MEDTECH
SERVICES
With a deep understanding of medical device regulations, quality standards, and product development processes, we provide tailored solutions to medical technology companies of all sizes. Whether you’re a startup or an established industry player, our experienced consultants offer guidance and support at every stage of your product lifecycle.
From regulatory compliance to market entry strategies, we help you navigate the intricate regulatory pathways and accelerate the time-to-market for your innovative medical devices. Our expertise spans diverse areas such as:
Regulatory Affairs (Submission and Compliance)
- CE Mark, Virtual Manufacturing (also known as Own Brand Labelling)
- FDA IDE, 510(k) & PMA Submissions
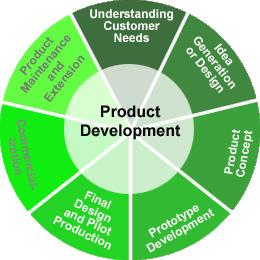
Product Design and Development
Clinical Evaluation Report
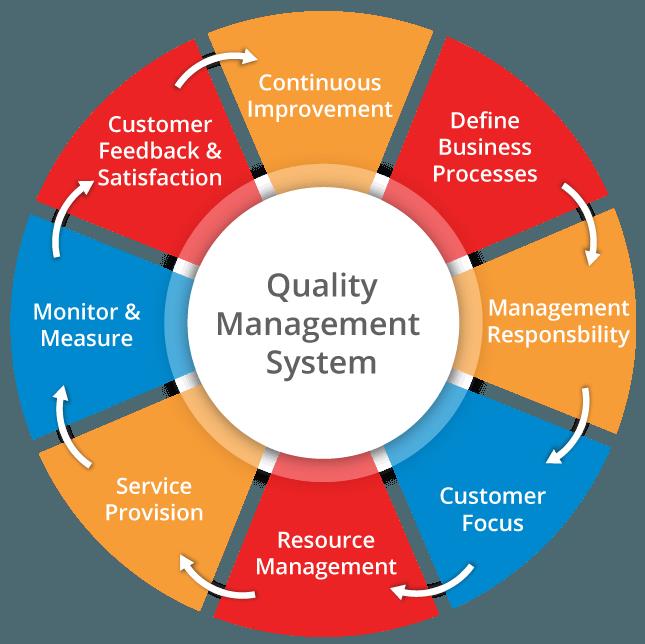
Quality Management Systems
- ISO 9001 (for Product and Service Quality Improvement)
- ISO 13485, FDA QSR, 21 CFR 820 (for Medical Device Regulatory Compliance)
Environmental Management System, ISO 14001
Risk Management as per ISO 14971
Project Management (ISO 21500)
Document Management Systems
Product Technical Documentation
Technical File and Design Dossier
Instructions For Use (IFU)
Operating Manuals preparations for Regulatory Compliance
Labelling Regulatory Compliance
- MDR
- UKCA
- US FDA
- etc…
Post Market Surveillance and Vigilance
Our Project and Programme Management approach combines the many good practices with Total Quality Management and Risk Management techniques. Our Project and Programme Managers work seamlessly with client organisations, teams, contractors and suppliers to deliver the agreed goals of any task undertaken on schedule. We aim to deliver our promise of "right first time" which is an integral part of our quality statement. Our Project and Programme Management typically considers the following contents depending on the dimensions and applicable features to achieve goals:
- Project Definition & Planning Resourcing and Mobilisation
- Project Structure & Organisation Process Control
- Documentation Control Risk Management
- Configuration Management Team Building, Collaboration & Effective Communication
- Quality Management Quality Audit
- Subcontractor and Contract Management Automated Project Management Tools
- Organisational Change Scope & Change Control
At Triune Tech we believe that "A project is not successful unless it delivers positive benefit to the organisation. It is not fully successful unless it delivers optimum benefit."
With diverse expertise in research and development in the medical and healthcare fields, we pride ourselves in Advanced Technology and Knowledge Transfer, transforming concepts into real innovative services and products.
Assurance:
Regulatory, Quality Management & Information Security Management Systems
We provide excellent services on consultation basis in the Quality Assurance and Management Systems. Our extensive knowledge on Quality Assurance Systems design, implementation and management is superlative. These robust systems will not only help your company meet Regulatory Authorities’ requirements but also optimise systems to maximise return on investment (ROI).
We provide comprehensive training across the board customising the system to meet your company’s needs. We design, implement and manage the system with regular evaluation to ensure continuous improvement of services and products. We also offer an integrated IT – Quality Assurance and Management Systems as we collaborate with other well-established eQMS providerd. We organise regular meetings with our clients to evaluate systems to check progress, identify practical cases which need corrective and preventive measures. These enhance efficiency and increase productivity.
At Triune Tech, we consider Business Assurance is the utmost factor in any organisation’s sustainability as it comprises of the concept that involves following best practices, implementing a flexible and effective control framework, monitoring risks and controls, auditing data and processes, managing contracts and compliance (regulations and legal), and improving quality integrity and customer experience. It is a data-centric, pro-active assurance umbrella framework that has evolved from over the year to include factors that affect the organisation’s sustainability.
Ultimately, business assurance is about providing organizations with increased confidence in their control environment and with improved efficiency of their business processes, maintaining reasonable assurance that they’re in control of their business.
We work to achieve and maintain assurances offered by the following systems:
- Regulatory Management Systems,
- Quality Management Systems
- Information Security Management Systems
With many years of regulatory experience with in-depth knowledge of regulatory requirements and keeping track of changes in legislations, directives/regulations, guidance documents and recommendations, we provide comprehensive solutions to help your product or services get to the global markets faster.